
Colour is defined as the visual sensations produced upon the retina by light waves of different lengths.
Light is defined as a form of energy which is radiated by means of electromagnetic waves measured in centimetres or nanometres which are equal to one millionth of a millimetre.
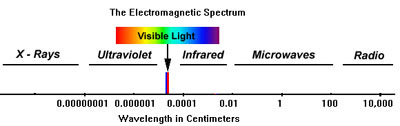
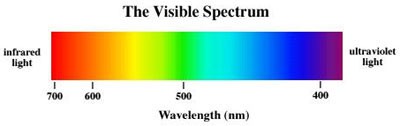
Is composed of an approximately equal mixture of all colours or wavelengths that make up the visible spectrum.
Red / Orange / Yellow / Green / Blue / Violet
A Coloured Gemstone in White Light
The colour we see is the result of the absorption by the stone of various wavelengths of the original white light.
Transparent Stones - absorption occurs as the light passes through the stone.
Opaque Stones - absorption occurs as the light is reflected from the stones surface.
Selective Absorption: The suppression of certain wavelengths or colours in white light. It is caused either by impurities present in the gemstone (i.e Chromium in Ruby or Iron in Amethyst) or by chemicals in the stones composition (i.e Copper in Malachite or Manganese in Rhodonite).
Allochromatic Gemstones: Gemstones whose colours are caused by impurities.
Idiochromatic Gemstones : Gemstones who owe their colour to their own chemical composition.
Selective absorption of light in both Allochromatic and Idiochromatic gems is caused mainly by the presence of "Transition Elements".
|
Vanadium |
Synthetic Corundum (Alexandrite Colour change), Blue/Violet Sapphire |
|
Chromium |
Ruby, Emerald, Alexandrite, Red Spinel, Jadeite,Demantoid Garnet, Pyrope Garnet, Pink Topaz |
|
Iron |
Amethyst, Sapphire, Peridot, Aquamarine, Tourmaline, Almandine Garnet |
|
Nickel |
Chrysoprase Quartz, Synthetic Green and Yellow Sapphires |
|
Manganese |
Rhodochrosite, Rhodonite, Spessartite Garnet, Rose Quartz |
|
Copper |
Malachite, Turquoise, Synthetic Green Sapphire |
|
Cobalt |
Synthetic Blue Spinel, Blue Synthetic Quartz, Cobalt Glass and Natural Blue Spinel |
|
Titanium |
Blue Sapphire |
Metamerism: Colour change effect seen when a stone is moved from one type of lighting to another (i.e Alexandrite).
In Alexandrite, there is a broad absorption band in the yellow part of the spectrum.
Alexandrite appears green by daylight since this light is rich in shorter wavelengths and red in artificial light (not fluorescent lighting) since this light is rich in longer wavelengths.
The tungsten lamp is blue-deficient hence the red colour seen in Alexandrite
The phenomenon termed "Selective Absorption" can be made visible by using an instrument called a Spectroscope. By using a series of prisms or diffraction grating, it is possible to analyze the light as it passes through a gemstone. The result is called an "Absorption Spectrum" in which the colours or wavelengths absorbed by the gemstone appear as dark bands.
Uses:
Disadvantages:
Costly.
Wavelengths are not linearly spaced out. The red end is bunched whilst the blue/violet is spread out.
Uses:
Same as the prism type spectroscope.
Disadvantages:
Spectrum is not as bright.
Hard to regulate the amount of light that enters the instrument.
Hard to view in the blue end of the spectrum.
Advantages:
Cost, they are relatively inexpensive.
Extremely portable.
Transition Elements :
|
Vanadium |
|
|
Chromium |
|
|
Iron |
|
|
Nickel |
|
|
Manganese |
|
|
Copper |
|
|
Titanium |
|
|
Cobalt |
|